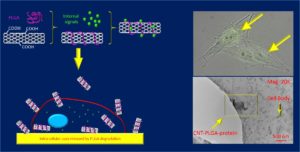
Cell morphodynamics and interactions with microenvironment
Human pluripotent stem cells (hPSCs) offer a promising tool for regenerative therapies because of their unique capability to self-renew, proliferate nearly indefinitely, and rise to various cell types. In the regeneration process, the temporary extracellular matrix (ECM) provides multiple signals to hPSCs and guides the process of new matrix formation. Fundamental questions on how hPSCs sense and respond to the cues from surrounding environment await further exploration. A main hurdle facing the studies of hPSC-ECM interactions lies in limitation of hPSCs culture techniques which use clumps of cells from dissected colonies. In these culture methods, hPSCs unavoidably pass through the stage of spontaneous differentiation to all three embryonic germ layers before the generation of the desired tissue-specific cell type. The goal of this study is to generate and characterize a monolayer single-cell culture model that allows for the investigation of biological phenomena associated with hPSC lineage specification. Specifically, we seek to (1) leverage micropatterning approaches to control cell shape and orientation, and activation of polarity proteins in hPSCs at a single cell level, and (2) quantitatively map the contribution of cell shape to germ layer specification of hPSCs.
Representative Publications:
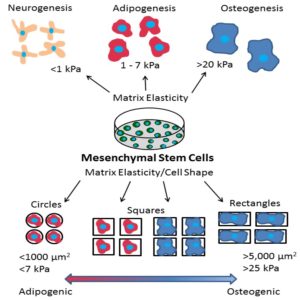
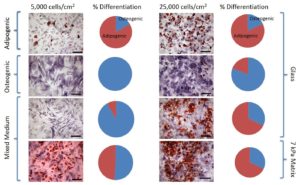
G. Harris, M. E. Piroli, and E. Jabbarzadeh, “Deconstructing the effects of matrix elasticity and geometry in mesenchymal stem cell lineage commitment,” Advanced Functional Materials 14: 2396-2403 (2014). Download PDF
G. Harris, T. Shazly, and E. Jabbarzadeh, “Deciphering the combinatorial roles of geometric, mechanical, and adhesion cues in regulation of cell spreading,” PLoS One 8(11) e81113 (2013). Download PDF